Our initial success with burns strongly suggested that the Cord Lining Stem Cells could also resurface chronic wounds and ulcers. The examples below show successful treatments we have achieved on ulcers and wounds where conventional treatments had failed. We hypothesised that the Cord Lining Stem Cells were secreting proteins that had modulated the chronic wound environment, making it amenable for repair.
Examples of successful treatments of wounds using our stem cell technology:
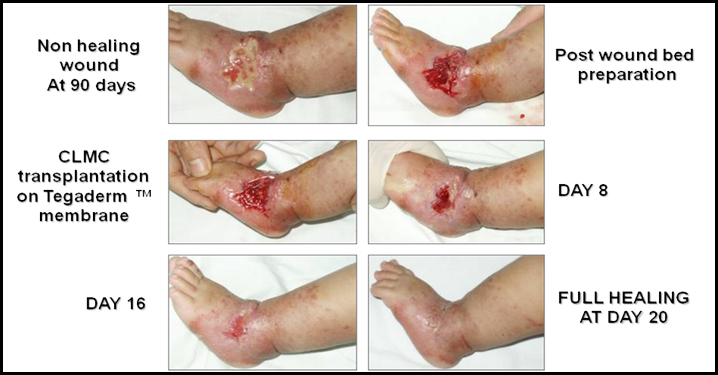
2 year old male with a chronic ulcer on the ankle after being treated for a blood vessel tumour, called a vascular malformation, with external irradiation. The vascular malformation shrank but a non-healing wound was left in its wake. Treatment with Cord Lining Stem Cells closed the wound in 20 days after it had failed to heal for 3 months.
CLSC treatment and healing of a chronic ulcer from external irradiation
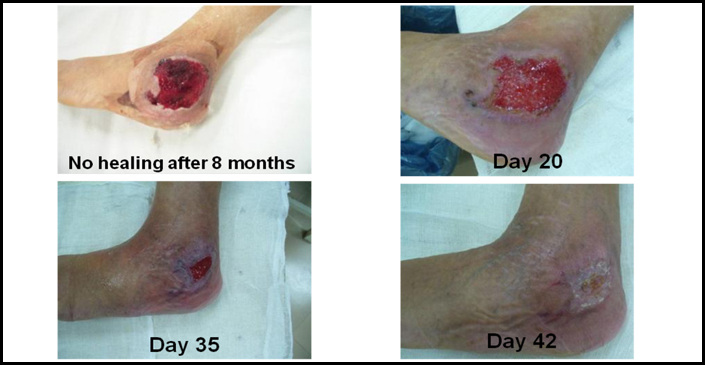
14-year-old male with a clotting disorder called Haemophilia. Patients with this condition are unable to clot their blood and can die from uncontrolled bleeding. In his history he bumped his elbow, which accumulated a blood clot (called a haematoma) compromising skin blood flow. The skin broke down revealing a large ulcer. His surgeons were unable to take a skin graft for fear he might bleed uncontrollably from where the skin graft was harvested (called the skin graft donor site). Treatment with Cord Lining Stem Cells healed the wound in 42 days without need for a skin graft.
CLSC treatment and recovery of a chronic ulcer in a patient with Haemophilia A
30-year-old female who sustained severe injuries in a road traffic accident when she was flung from a motorcycle, sustaining forearm fractures and tissue loss (an open fracture). Bone was exposed and skin grafting on bone was not feasible so her surgeons suggested amputation. Fearful of losing her forearm, this young lady opted to use Cord Lining Stem Cells, which successfully closed the wound over the exposed bone after 21 days, saving her from a forearm amputation.
CLSC treatment and healing of a complex open forearm wound with exposed bone
CellResearch is actively conducting chronic wound resurfacing applications using Cord Lining Stem Cells with these clinical collaborators:
Dr. Mai Manh Tuan at St Paul’s Hospital Burns Centre and the National Hospital of Traditional Medicine in Hanoi, Vietnam;
Dr. Dinh Van Han at Hospital 103 in Hanoi, Vietnam
The outstanding efficacy of Cord Lining Stem Cells in the healing of wounds has spurred CellResearch Corporation to accelerate development in this area, and as of 2012 we started looking into starting a USFDA trial to qualify the efficacy of this treatment, and to allow its use in the general patient population. The trial centre has now been finalised to be The University of Colorado Anschutz Medical Campus with our research collaborator Clinimmune Labs, and the University of Colorado. The IND (Investigational New Drug) application has been approved and we will shortly begin Phase I of the trial!
cGMP Cord Lining Stem Cells
Cells produced for human clinical therapy are produced under the most stringent conditions (Current Good Manufacturing Practice- cGMP) in order to ensure the highest levels of quality and safety. CellResearch are pleased to announce that by 2020, cGMP grade Cord Lining Stem Cells will be produced in Singapore suitable for use in human clinical trials which can therefore start by that time. There are 2 clinical trials awaiting these cells in Singapore.
cGMP grade Cord Lining Stem Cells will be produced by Clinimmune Labs for use in the USFDA trial.